61medical develops solutions from the clinical problem to approved medical products according to ISO standards and MDD/MDR. Our proprietary solutions or our contractual developments can be handled in work packages, going as far as 61medical acting as the legal manufacturer.
61medical benefits from many areas of expertise along its development process.
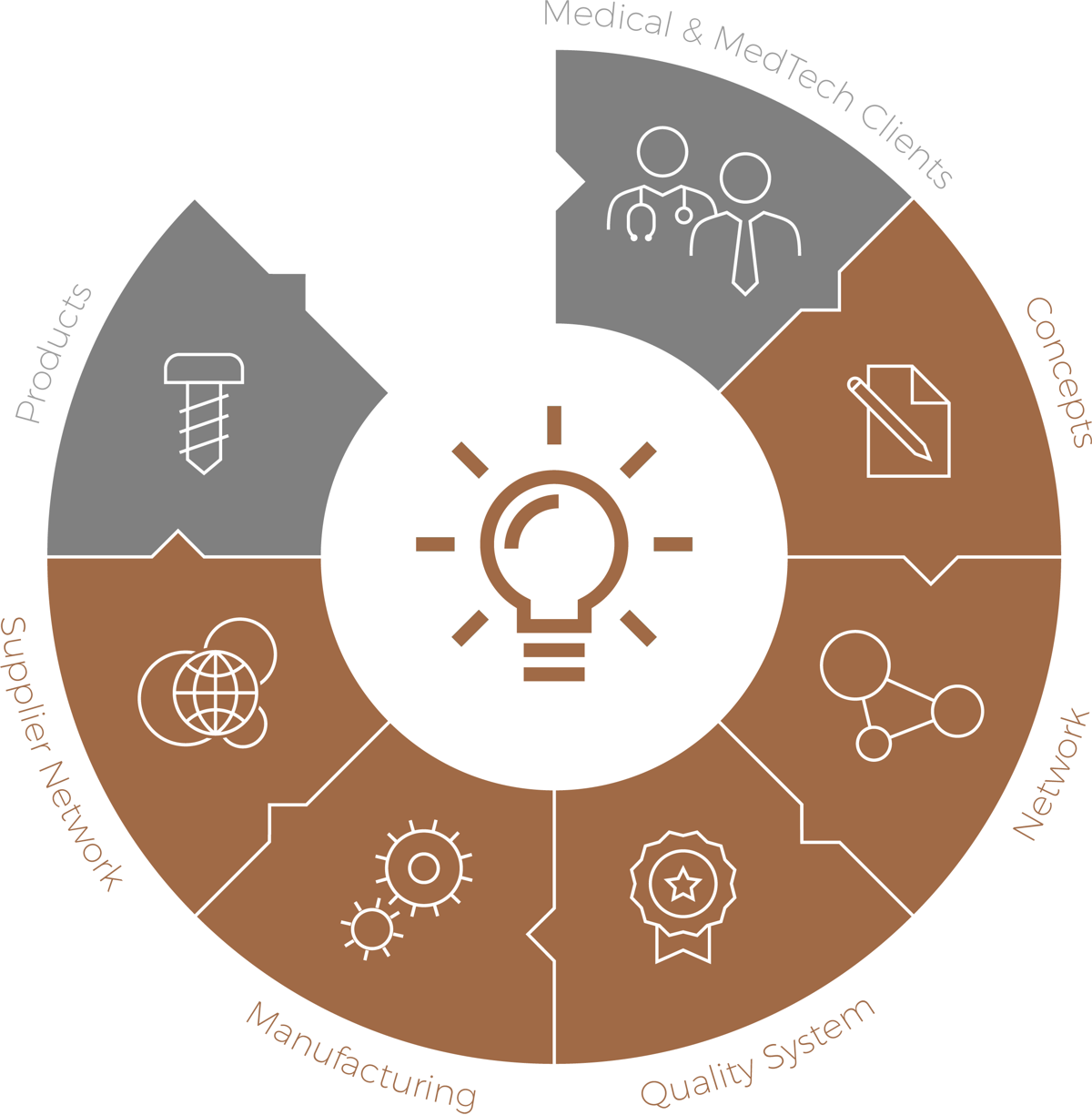
At 61medical, the innovation and lean process PRAGMATISIMO™ for a new product is handled in flexible work packages that can be individually purchased and ended after each phase. R&D, manufacturing and quality engineers are involved as a cross functional team along the whole product development process.